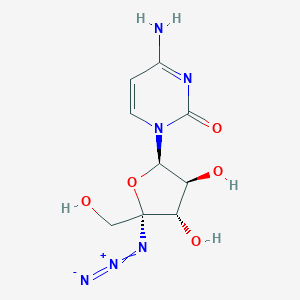
-
RO-9187
- names:
RO-9187
- CAS号:
876708-03-1
MDL Number: MFCD22665689 - MF(分子式): C9H12N6O5 MW(分子量): 284.23
- EINECS: Reaxys Number:
- Pubchem ID:11514721 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM000579-10mg | 10mg | >98.0% | ¥ 3389.00 | ¥ 3389.00 | 2-3天 | ¥ 0.00 | ||
| YZM000579-5mg | 5mg | >98.0% | ¥ 2494.05 | ¥ 2494.05 | 2-3天 | ¥ 0.00 |
| 中文别名 | RO-9187(cas:876708-03-1),RO9187,RO 9187 |
| 英文别名 | RO-9187(cas:876708-03-1),RO9187,RO 9187,4-Amino-1-(5-azido-3,4-dihydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-1H-pyrimidin-2-one |
| CAS号 | 876708-03-1 |
| Inchi | InChI=1S/C9H12N6O5/c10-4-1-2-15(8(19)12-4)7-5(17)6(18)9(3-16,20-7)13-14-11/h1-2,5-7,16-18H,3H2,(H2,10,12,19)/t5-,6-,7+,9+/m0/s1 |
| InchiKey | ODLGMSQBFONGNG-XZMZPDFPSA-N |
| 分子式 Formula | C9H12N6O5 |
| 分子量 Molecular Weight | 284.23 |
| 溶解度Solubility | 生物体外In Vitro:H2O : 7.14 mg/mL(25.12 mM;Need ultrasonic) |
| 性状 | 固体粉末,Power |
| 储藏条件 Storage conditions | -20°C 3 years年 4°C 2 years年 / In solvent溶液中:-80°C 6 months月 -20°C 1 month月 |
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tag:RO-9187蒸汽压,RO-9187合成,RO-9187标准,RO-9187应用,RO-9187合成,RO-9187沸点,RO-9187闪点,RO-9187用途,RO-9187溶解度,RO-9187价格,RO-9187作用,RO-9187结构式,RO-9187用处
| 产品说明 | RO-9187(876708-03-1)是高效的HCV病毒复制的抑制剂,IC50值是171 nM |
| Introduction | RO187(876708-03-1) is a potent inhibitor of HCVvirus replication with anIC50of 171 nM. |
| Application1 | |
| Application2 | |
| Application3 |
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303吞入可能有害+H313皮肤接触可能有害+H2413吸入可能对身体有害 |
| 安全防护 | P264处理后彻底清洗+P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具+P305如果进入眼睛+P351用水小心冲洗几分钟+P338取出隐形眼镜(如果有)并且易于操作,继续冲洗+P337如果眼睛刺激持续+P2393获得医疗建议/护理 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| 2012-03-01 Defibrotide interferes with several steps of the coagulation-inflammation cycle and exhibits therapeutic potential to treat severe malaria Arteriosclerosis, thrombosis, and vascular biology |
| 2009-05-14 The design, synthesis, and antiviral activity of monofluoro and difluoro analogues of 4'-azidocytidine against hepatitis C virus replication: the discovery of 4'-azido-2'-deoxy-2'-fluorocyt |
| 2009-01-08 The design, synthesis, and antiviral activity of 4'-azidocytidine analogues against hepatitis C virus replication: the discovery of 4'-azidoarabinocytidine Journal of medicinal chemistry |
| 2008-01-25 2'-deoxy-4'-azido nucleoside analogs are highly potent inhibitors of hepatitis C virus replication despite the lack of 2'-alpha-hydroxyl groups The Journal of biological chemistry |
| 2'-deoxy-4'-azido nucleoside analogs are highly potent inhibitors of hepatitis C virus replication despite the lack of 2'-alpha-hydroxyl groups PMID 18003608; The Journal of biological chemistry 2008 |
Abstract:Tick-borne encephalitis virus (TBEV) causes a severe and potentially fatal neuroinfection in humans. Despite its high medical relevance, no specific antiviral therapy is currently available. Here we demonstrate that treatment with a nucleoside analog, 7-deaza-2'-C-methyladenosine (7-deaza-2'-CMA), substantially improved disease outcomes, increased survival, and reduced signs of neuroinfection and viral titers in the brains of mice infected with a lethal dose of TBEV. To investigate the mechanism of action of 7-deaza-2'-CMA, two drug-resistant TBEV clones were generated and characterized. The two clones shared a signature amino acid substitution, S603T, in the viral NS5 RNA-dependent RNA polymerase (RdRp) domain. This mutation conferred resistance to various 2'-C-methylated nucleoside derivatives, but no cross-resistance was seen with other nucleoside analogs, such as 4'-C-azidocytidine and 2'-deoxy-2'-beta-hydroxy-4'-azidocytidine (RO-9187). All-atom molecular dynamics simulations revealed that the S603T RdRp mutant repels a water molecule that coordinates the position of a metal ion cofactor as 2'-C-methylated nucleoside analogs approach the active site. To investigate its phenotype, the S603T mutation was introduced into a recombinant TBEV strain (Oshima-IC) generated from an infectious cDNA clone and into a TBEV replicon that expresses a reporter luciferase gene (Oshima-REP-luc2A). The mutants were replication impaired, showing reduced growth and a small plaque size in mammalian cell culture and reduced levels of neuroinvasiveness and neurovirulence in rodent models. These results indicate that TBEV resistance to 2'-C-methylated nucleoside inhibitors is conferred by a single conservative mutation that causes a subtle atomic effect within the active site of the viral NS5 RdRp and is associated with strong attenuation of the virus.IMPORTANCE This study found that the nucleoside analog 7-deaza-2'-C-methyladenosine (7-deaza-2'-CMA) has high antiviral activity against tick-borne encephalitis virus (TBEV), a pathogen that causes severe human neuroinfections in large areas of Europe and Asia and for which there is currently no specific therapy. Treating mice infected with a lethal dose of TBEV with 7-deaza-2'-CMA resulted in significantly higher survival rates and reduced the severity of neurological signs of the disease. Thus, this compound shows promise for further development as an anti-TBEV drug. It is important to generate drug-resistant mutants to understand how the drug works and to develop guidelines for patient treatment. We generated TBEV mutants that were resistant not only to 7-deaza-2'-CMA but also to a broad range of other 2'-C-methylated antiviral medications. Our findings suggest that combination therapy may be used to improve treatment and reduce the emergence of drug-resistant viruses during nucleoside analog therapy for TBEV infection.
2.Antiviral candidates against the hepatitis E virus (HEV) and their combinations inhibit HEV growth in in vitro/PMID 31362004; Antiviral research 2019 10; 170(?):104570/Name matches: 2'-c-methylguanosine ro-9187
Abstract:Hepatitis E is a global public health problem. Ribavirin (RBV) and pegylated interferon alpha are currently administered to cure hepatitis E. Recently, in combination with RBV, sofosbuvir (SOF), an anti-hepatitis C virus nucleotide analog, is also given to patients with chronic hepatitis E. However, this combinatorial therapy sometimes fails to achieve a sustained virological response. In this study, we used 27 antiviral compounds, including 15 nucleos(t)ide analogs, for in vitro screening against a genotype 3 HEV strain containing a Gaussia luciferase reporter. RBV, SOF, 2'-C-methyladenosine, 2'-C-methylcytidine (2CMC), 2'-C-methylguanosine (2CMG), and two 4'-azido nucleoside analogs (R-1479 and RO-9187) suppressed replication of the reporter genome, while only RBV, SOF, 2CMC and 2CMG inhibited the growth of genotype 3 HEV in cultured cells. Although 2CMG and RBV (2CMG/RBV) exhibited a synergistic effect while SOF/RBV and 2CMC/RBV showed antagonistic effects on the reporter assay, these three nucleos(t)ide analogs acted additively with RBV in inhibiting HEV growth in cultured cells. Furthermore, SOF and 2CMG, with four interferons (IFN-α2b, IFN-λ1, IFN-λ2 and IFN-λ3), inhibited HEV growth efficiently and cleared HEV in cultured cells. These results suggest that, in combination with RBV or interferons, SOF and 2CMG would be promising bases for developing anti-HEV nucleos(t)ide analogs.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻
怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33
提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07
各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28
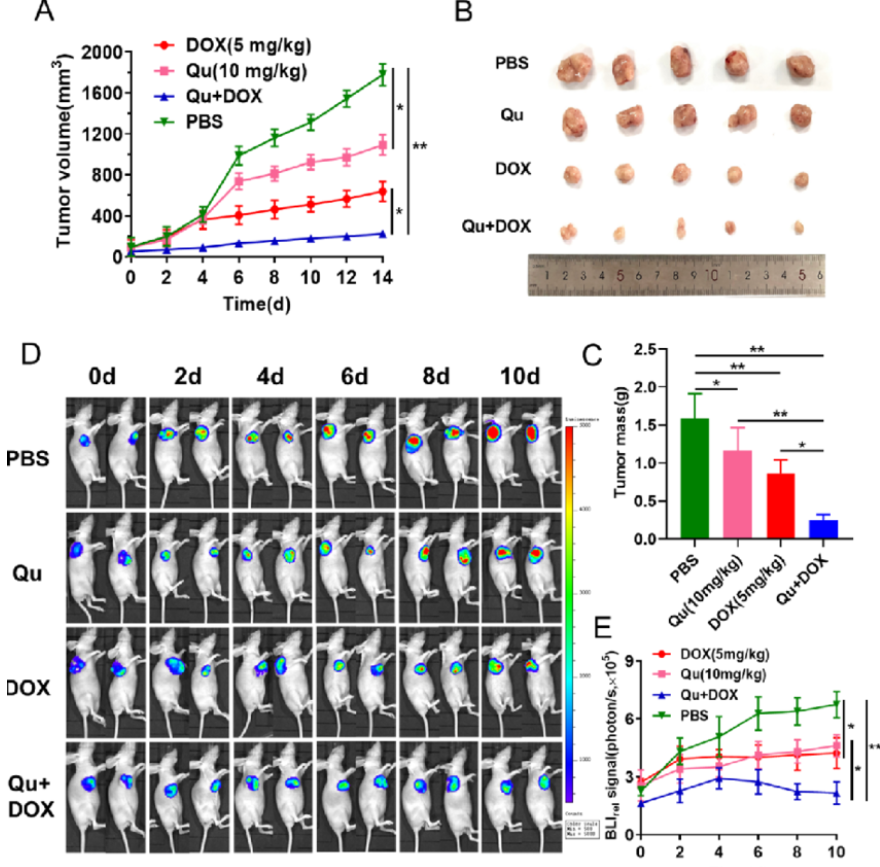
荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11
如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



